Chemistry notes
Chemistry notes
The following text is used only for teaching, research, scholarship, educational use and informative purpose following the fair use principles.
We thank the authors of the texts and the source web site that give us the opportunity to share their knowledge
Chemistry
Chemistry notes
Classification of Matter
One technique which makes the task of the scientist easier is to provide classification schemes in order to categorize seemingly similar or widely diverse things.
It is important to note that any classification scheme is arbitrary, and there may be another scheme, which is just as valid. In some cases, scientists agree on a scheme, which makes it easier to communicate information and ideas. This is certainly true for the classification of plants and animals.
Let us begin by considering some of the major types of chemical substances.
- IMPURE SUBSTANCES
These have two or more components, which may be mixed in any proportions. These can be mechanically separated by a process, which does not require a chemical reaction such as filtering, centrifuging, settling, etc. There are two general types of mixtures.
- Homogeneous mixtures. These are usually called solutions that are the result of a combination of phases. Examples include sugar in water, (solid in liquid), air (gas in gas), carbonated water (gas in liquid), and alloys like brass or stainless steel (solid in solid). Acids and bases are other solutions, which have unique properties. Although they are homogeneous, all solutions can be separated by some physical means such as evaporation, boiling etc.
- Heterogeneous mixtures. These are NOT uniform throughout and are the easiest to separate. Some may separate spontaneously such as oil and vinegar salad dressing, which has to be shaken before use.
- PURE SUBSTANCES
These have only one ingredient. They cannot be separated into simpler components by mechanical (physical) methods such as filtering, boiling, centrifuging, etc.
- Elements. These are made up of only one type of atom. There are about 107 elements presently known. Some of them are metals, and others are nonmetals. All the known elements are classified with another scheme - the periodic table, which will be studied in detail in the next part of this unit. You may refer to the periodic table where you will see the division between metals and nonmetals by the stepwise line starting at the element boron.
- Compounds are made up of two or more different elements, but are pure substances because they consist of only one kind of molecule or formula unit. Theoretically, an infinite number of compounds are possible. New compounds are being continuously discovered, and this leads to some exciting research in chemistry, especially in the plastics, and other synthetic products industries. The two general types of compounds are:
- Covalent compounds. These are made up of two or more nonmetallic elements, bonded together by sharing electrons to form covalent bonds. Examples of common covalent molecules are carbon monoxide (CO), sulfur dioxide, and air pollutant (SO2), carbon dioxide (CO2), and table sugar (C12H22O11).
- Ionic compounds. These generally consist of a metal and a nonmetal which through the exchange of electrons, have become positively and negatively charged. These charged particles are called ions and are attracted to each other due to their opposite charges. Examples are ordinary table salt, sodium chloride (NaCl), and road salt to melt ice, calcium chloride (CaCl2).
Exercise
Suggest how each could be separated into its components.
- iron filings and powdered sulfur
- table salt and water
- sand, sugar and water (well mixed)
- blood
- crude oil
- alcohol and water
AN ELEMENTAL TALE: THE GOLD DUST KID
The Kid mounted his trusted steed, old (B) ________ . His shooting (Fe) ________ strapped to his side, he headed out for the bright (Ne) ________ lights of Sabuttus, aiming to rob the Litchfield stage. There was sure to be a load of precious (U) ________ aboard, and probably (K) ________ too. Inhaling a deep breath of (O) ________ he coughed on the (S) ________ from the nearby mills. Since the (Hg) ________ was climbing, he quenched his thirst with some H2O, tasting the (Cl) ________ all big cities like Wales had. As he headed north, his riding bones ached from (Ca) ________ deposits built up over years of riding the (Zn) ________ trail. Overhead a (He) ________ - filled balloon floated in the breeze; the sun beat down like burning (P) ________ . Soon he spotted the stage, guarded only by a sheriff with a (Sn) ________ badge. "Halt", he yelled, "or I'll fill you full of (Pb) ________ ." The sheriff drew his gun, but alas, was too slow. The Kid's gun, blazing like flaming (Mg) ________ did the (Cu) ________ in. Anyone who drew on the Kid should know his life wasn't worth a plugged (Ni) ________ . A (Pt) ________ blond riding beside the (Al) ________ - framed coach rode for her life when the Kid pulled out some (N) ________ compounds, preparing to blow the safe to atoms. Suddenly, a shout rang out, "Hi Ho (Ag) ________ !" and a masked man on a white horse raced across the (Si) ________ sands like (Na) ________ skittering on H2O. A (H) ________ bomb would not have stopped the lawman; the Kid had met his doom. The rest of his life was to be spent behind (Co) ________ steel bars, a warning to all who flirt with danger. Your detention may be the initial step in a (C) ________ copy life of the saga of the (Au) ________ Dust Kid.
The Periodic Table
Dmitri Ivanovich Mendeleev, (1834-1907), a Russian chemist, was fascinated by the problem of organizing the elements in a table. He noted that when lithium, magnesium, boron, carbon, nitrogen, oxygen, and fluorine were arranged in order of their atomic masses, the order of their valences repeated itself when the next seven elements were arranged in order of their atomic masses. Mendeleev arranged the 63 elements known at that time into a table in order of their atomic masses so that elements with the same valence appeared in the same row, and called it a Periodic Table of the elements.
There were a number of blank spaces in Mendeleev's first periodic table. Instead of regarding the blanks as an inadequacy of his arrangement, he boldly predicted that new elements would be discovered to fill in the blanks. He even predicted some of the properties of the missing elements. Mendeleev published his periodic table in 1869. Scientists were not impressed at first, but when some of the elements he had predicted were actually discovered, he became the most famous chemist in the world. His periodic table eventually led to the discovery of 23 elements in the three decades following its publication.
Exercise
- What is periodic about the Periodic Table?
- What were Mendeleev's two ordering principles (horizontal and vertical)?
- How did Mendeleev explain the blank spaces on his periodic table?
Lab A5: Properties and Applications of Elements
Go to the 15 stations provide and observe and classify each pure element. Design a suitable data table to record each element's name, symbol, color, luster, ductility, conductivity, and magnetic attraction and whether it is a metal or nonmetal. Leave each station organized as you found it.
More Questions on the Elements
- Three metals which are commonly used for plating alloys or other metals are
a)
b)
c)
- The three elements which are magnetic are
a)
b)
c)
- Aluminum, chromium, and stainless steel listed in order of brilliance from dullest to brightest are
a)
b)
c)
- The two elements with the greatest densities are __________ and __________ . (Find the actual densities on the periodic table.)
- The two elements with the smallest densities are __________ and __________ .
- The element(s) that can not be classified as either metal or nonmetal based on their properties are (metaloids)____________________.
- Differentiate between an oxide, ore, alloy, and plated metal by defining the following terms.
- oxide
- ore
- alloy
- plated metal
ELEMENTS AND THE PERIODIC FAMILY
BACKGROUND INFORMATION FOR THE ELEMENTS
Element |
Discovery or Isolation |
Sources |
Alloys |
Uses |
Comments |
||
Chemist |
Year |
World |
Canada |
||||
phase s/l/g |
ancients |
- |
2. U.S.A
|
|
Ag-Cu and
(Ag-Cu)
(Ag-Cu) |
(92.5% Ag, 7.5% Cu)
- batteries (Ag-Zn) |
|
phase s/l/g |
ancients |
- |
|
Negligible mining and no processing |
Solder |
|
1/2% tin in a tin can (tinplated) |
phase s/l/g |
Marggraf |
1746 |
|
|
Brass |
|
|
4. magnesium, |
Davy |
1808 |
|
- mine and smelter (80km W of Ottawa) |
Mg-Al |
Mg-Al alloys
|
|
5. sulfur, S8 |
ancients |
- |
4. Poland |
|
|
|
|
Element |
Discovery or Isolation |
Sources |
Alloys |
Uses |
Comments |
||
Chemist |
Year |
World |
Canada |
||||
phase s/l/g |
ancients |
- |
5. Australia |
|
(0.1% to 0.3% C)
|
|
|
7. copper, Cu |
ancients |
- |
|
province |
(Cu, Zn, Sn)
(Cu, Zn, Ni)
(Ag, Cu) |
|
|
Hg |
ancients |
- |
|
|
|
|
|
9. carbon, C |
ancients |
- |
|
|
Steels
|
|
|
Element |
Discovery or Isolation |
Sources |
Alloys |
Uses |
Comments |
|||
Chemist |
Year |
World |
Canada |
|||||
Al |
Hans Oersted |
1825 |
|
|
Duralumin for aircraft |
|
|
|
Cr |
Vauquelin |
1798 |
|
No mine production from Canadian deposits |
|
|
|
|
12. lead, Pb |
ancients |
- |
|
|
Lead type |
|
|
|
Element |
Discovery or Isolation |
Sources |
Alloys |
Uses |
Comments |
|||
Chemist |
Year |
World |
Canada |
|||||
13. nickel, Ni |
Cronstadt |
1754 |
2. New
|
Refinery at fort Sask. Alberta |
|
(eg 8% Ni)
|
|
|
Ca |
Davy |
1808 |
No others in non-communist world |
|
|
|
|
|
W |
d'Elhuyer brothers |
1783 |
1. China
|
Trail, B.C. |
|
|
|
|
Use the periodic table, Lab A5 observations, information given in this unit, and everyday experience to provide the name and symbol for the element(s) which best fits the description or application. Some elements are used more than once in the exercise.
Description or Application |
Name and Symbol |
Description or Application |
Name and Symbol |
Eg pencil lead |
carbon, C |
Eg Canadian nickels |
nickel, Ni |
1. light bulb filament |
|
to steel alloys |
|
|
|
|
|
|
|
blackpowder (gunpowder) |
|
4. flashbulb filament |
|
|
|
5. foil wrap |
|
25. sterling silver |
|
6. household water pipes |
|
|
|
7. shiniest common metal |
|
26. common solder |
|
|
|
|
|
9. most used metal |
|
27. brass |
|
10. dry cell battery |
|
|
|
electricity |
|
steel to make stainless |
|
12. charcoal briquettes |
|
|
|
13. most abundant metal |
|
steel |
|
pipes |
|
|
|
natural gas |
|
elements |
|
16. stored in water |
|
|
|
17. plating on most cans |
|
|
|
18. common liquid metal |
|
elements |
|
lights |
|
|
|
20. poisonous yellow gas |
|
|
|
elements |
|
32. a yellow, solid nonmetal |
|
|
nonmetal |
|
|
elements |
|
34. a yellow, solid metal |
|
|
|
||
History
The Atom (indivisible)
Greeks » 400B.C. "qualitative thinkers"
Water
- change water to air
4 Fundamental Elements and Their Properties
The "Natural Place" of the Elements
The 5th element "Quintessence" that make up the heavenly bodies

FIRE

AIR

WATER

EARTH
* burning log
The Greek poem "De Rerum Natura" (On The Nature of Things) described atomism.
(Atom = Greek for indivisible)
Example cut up paper

The idea of the atom fell out of favor. Example: Pythagoras found it to be atheistic.

1800 - John Dalton
- "meteorologist" studied the weather.
- In order to understand the atmosphere, he experimented with gases.
- These experiments led him to believe in atoms.
- Developed a "billiard ball" model.
1899 - J. J. Thompsom
- Discovered the electron.
- Thus discovered the atom is "divisible".
- Developed a "raisin bun" model.
1914 - Bohr
- Suggested electrons were going around the nucleus like planets going around the sun.
- "Planetary Model"
1932 - Chadwick
- Discovers the neutron.
The Atom Example Helium
- According to the planetary model, electrons orbit the nucleus like planets orbiting the sun.
Overview - Models of the Atom
In 1804, John Dalton used a table of atomic masses as evidence for the theory that all substances are composed of atoms - the smallest particles of matter. Scientists such as Michael Faraday and J.J. Thomson (the electron), E. Goldstein (the proton), and James Chadwick (the neutron), were later to provide evidence to show that the atom is composed of 3 basic subatomic particles. Dalton's concept of the atom was to be progressively refined through the J.J. Thomson Model, the Rutherford (Nuclear) Model, the Bohr Model, and now the Quantum Mechanical Model of the atom.



 e-
e-

![]()
![]() + - - - 2e-
+ - - - 2e-
![]()
![]() - + 3+ 3+
- + 3+ 3+
![]() + - -
+ - -
Dalton Thomson Rutherford Bohr
(billiard ball) (raisin bun) (nuclear) (orbits)
(1804) (1900) (1912) (1913)
Models of the Atom
The progressive development of models of the atom is an interesting study of the manner in which all theories and models are developed. In general, a theory is developed to explain why nature behaves the way it does. A good model, arising from a theory, will be able to make predictions which will agree with future observations. (Mendeleev's periodic table is an example of a good model.)
Models of the Atom
By about 1800 a number of important generalizations existed which were developed from hundreds of experimental observations.
- The total mass of a system remains constant.
- Elements react in definite proportions.
- Compounds have a definite composition no matter how they are prepared.
These generalizations (i.e. laws), which are still valid today, provided a specific set of facts with which each progressive model must agree.
Dalton Model
According to Dalton, an element was composed of identical, invisible atoms. (The Dalton atom is commonly referred to as a billiard ball model.) The atoms of different elements had different properties (eg. mass). Molecules were explained as "compound atoms" made up of atoms of different elements in a simple whole number ratio. Dalton also devised a system of atomic symbols, wrote structural formulas and balanced equations. Dalton's theory and model correctly predicted the law of multiple proportions - if the same elements combine to form different compounds, the ratio of the elements in the compounds are in simple multiples.
The Dalton Model existed for over one hundred years. In spite of its success, the discovery of the subatomic particles and the study of radioactivity showed that atoms were not indivisible.
Thomsom Model
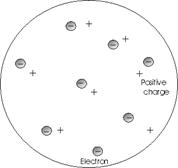

J.J. Thomsom revised the Dalton Model by proposing that the atom be considered a sphere of positive electricity in which negative electrons are embedded like raisins in a bun. It should be noted that the Thomson model did not alter many points of Dalton's theory concerning elements and their reactions to form compounds. Thomson postulated that most of the mass of the atom is associated with the positive electricity. This agrees with the observation that the positive fragments of atoms are much heavier that the electrons.
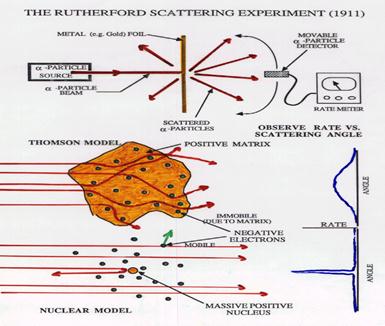
A classic experiment performed by Rutherford in 1911 showed that small positive particles are widely scattered as they pass through a thin gold foil. This evidence did not agree with a uniform distribution of mass and charge as is shown in the Thomson model and led to a further revision of the atomic model.
Rutherford Model
In the experiment mentioned above, Rutherford found that 99 percent of the atoms "shot" at the thin gold foil went straight through, some were deflected at large angles and a few were actually reflected back along their path.
Rutherford proposed that an atom has a nucleus or center in which it's positive charge and mass are concentrated. Almost the whole volume of the atom would be empty space occupied only by the moving negatively charged electrons. This nuclear model agreed with Rutherford's experimental findings in addition to the observations existing at this time (eg. radioactivity). Rutherford also postulated the existence pf neutrons (uncharged nuclear particles discovered in 1932 by Chadwick). The Rutherford model helped considerably in the understanding and progress of the periodic table of the elements.


During the time of Rutherford's work, many advances were made in the physics dealing with charged particles and radiation. This created a number of problems with the Rutherford Model. If the electrons are not moving, then the attraction of the negative electrons for the positive nucleus should collapse the atom. However, if they are in motion (to counteract the pull of the nucleus) then the electrons should radiate energy and spiral down to the nucleus. Obviously this does not happen since atoms, by and large, are very stable.
Bohr Model
About 1912, several scientists, including Neils Bohr, studied atomic spectra of the elements and discovered that each spectrum showed a series of lines of definite energies. To explain this observation (which did not agree with the Rutherford Model) and to explain why atoms do not collapse, Bohr assembled a new model of the atom. Bohr proposed that electrons of specific energy moved in circular orbits around the central atomic nucleus and that electrons could not exist between the orbits. This theory of electron motion may be compared, in a simplistic way, to the motion of the planets around the sun. The atomic spectrum of hydrogen and the periodic repetition of properties of the elements are two examples of experimental observations supporting the Bohr model.

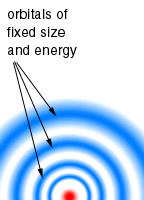
Even though the Bohr model was a tremendous step forward and still is a useful model, it was soon realized that properties of atoms more complex than hydrogen could not be explained exactly by this model.
Neils Bohr
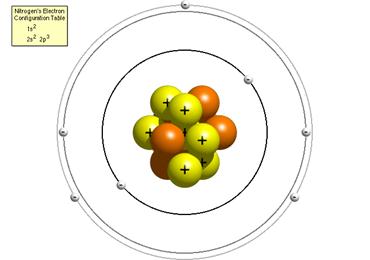
Bohr Model of Nitrogen
Quantum Mechanical Model
Advances in theoretical physics in the 1920's led to the development of the Quantum Mechanical Model. This model was a further refinement although the concept of electrons located in specific energy levels was still retained. Electrons are considered to have wave properties and occupy orbitals. The orbitals, surrounding the nucleus, represent probability patterns of the position of the electrons. Therefore, an electron is described in terms of its orbital rather than in terms of its orbit on a defined path.
The Quantum Mechanical Model is the most recent model and has to a large part overcome the pitfalls of the Bohr model. It's drawback is complexity - most mathematical equations arising from this model cannot be solved exactly by even the most powerful computers. Finally, the progressive development of atomic structure has now brought us to the point where the model is no longer a physical model but a complex mathematical model.
Summary
In the development of models of the atom, from the Dalton Model to the Quantum Mechanical Model, we have seen a progression where each successive model further refined an existing model. It should be noted that a good deal of chemistry we do today is and /or could be done using the Dalton Model.
Questions
- What are the features of a good theory?
- Should a theory be discarded if conflicting evidence is gathered?
WORKSHEET #7 - Exercise
- Michael Faraday and J.J. Thomsom are noted for their discovery of __________ .
- The proton was discovered by __________ .
- The neutron was discovered by __________ .
The concept of the atom was progressively refined as new evidence was discovered experimentally.
The next five questions are based on atomic theories.
- The theory that all matter was composed of unbelievably small particles called atoms was proposed by the Greek philosophers and revived in 1804 by __________ .
- The theory that an atom was a positively charged mass in which negatively charged electrons were embedded like "plums in a plum pudding" was suggested by __________ .
- The Nuclear Model for atoms where electrons surrounded a small massive nucleus was suggested in 1912 by __________ .
- The theory that electrons move around the nucleus of an atom in specific energy levels and where the atom was pictured as a miniature solar system was proposed in 1913 by __________ .
- The present model of the atom in which electrons occupy probability regions called orbitals is known as the __________ model.
- The smallest particle of matter (first proposed by the Greeks and then by Dalton) is known as an __________ .
- A neutral atom contains an equal number of __________ and __________ .
- An isotope of an element has an equal number of __________ and __________ but a different number of __________ .
- Electrons found in the outermost energy level of an atom are known as __________ electrons.
- A small but massive structure in the centre of an atom is called the __________ consisting of __________ and __________ .
- The __________ __________ of an element is equal to the number of protons located in the nucleus or the number of electrons surrounding the nucleus in a neutral atom.
- The protons and neutrons in an atom contribute most to the __________ __________ of a particular element.
- The elements in the periodic table are arranged horizontally in order of increasing __________ __________ .
- Each period, except the first, starts with a __________ family and ends with the __________ family.
- The heavy staircase line divides the __________ from the __________ .
- Vertical arrangements of elements in the periodic table are called __________ or __________ .
- The most metallic element is __________ and the most nonmetallic element is __________ .
- The period number of an element equals the number of __________ __________ occupied by electrons.
WORKSHEET #8 - Questions: Overview of Periodicity and Atomic Structure
- The extranuclear region of the atom, which makes up most of the volume of the atom, is occupied by __________ .
- Nearly all of the mass of any atom is made up of __________ and __________ .
- Elements 58 through 71 and 90 through 103 are called the __________ and __________ .
- S 2- would have __________ electrons around its nucleus.
- And atom has 53 protons in its nucleus. In a neutral atom it will also have __________ electrons and it will (gain/lose) __________ electron(s) to acquire the electron population of the nearest noble gas, __________ .
- Elements 4, 12, and 20 are closely related chemically. The name of one other element which would fit into this family called the __________ is __________ .
- Element 19 has one electron taken from it. The symbol for its ion is __________ .
- An unknown element is a colorless gas at room temperature. Upon heating with lithium, no reaction occurs. The family of elements to which this unknown element probably belongs is __________ .
- A soft metal reacts vigorously with water to produce hydrogen gas, H2. This metal probably belongs to the __________ family.
- The most reactive metal is __________ and the most reactive nonmetal is __________ .
- The elements which make up the B groups on the periodic table are called the __________ elements.
- The number of electrons in the third energy level of a chlorine atom is __________ .
- The atomic number of a K atom is (greater/less) than the atomic number of a Na atom.
- The scientist who first proposed that electrons existed in only certain energy levels about the nucleus was __________ .
- The name of the ion formed by a bromine atom is __________ .
- The name of the ion formed by a calcium atom is __________ .
- The number of __________ in the nucleus of chlorine atoms may vary.
- The number of electrons in the outermost energy level of a potassium ion is __________ .
- Atoms with the same number of protons but with a different number of neutrons in the nucleus are called __________ .
- The average mass of atoms for a particular element is called the __________ __________ .
- The scientist who proposed the Nuclear Model of the atom was __________ .
- The scientist who introduced the word atom and used experimental evidence to present the Atomic Theory was __________ .
- The maximum numbers of electrons in the first three energy levels are respectively __________ , __________ , and __________ .
- The charges (and their magnitudes) on simple ions formed from atoms in groups IA, IIA, IIIA, VA, VIA, and VIIA are respectively __________ , __________ , __________ , __________ , __________ , __________ .
COMPOUNDS, BONDING, AND NOMENCLATURE
INTRODUCTION
Classification of Substances
Recall the classification of substances flowchart in the previous unit.
The first unit concentrated on the classification of elements. In this unit, attention is focussed on the classification of compounds. In addition, this unit contains a study of the properties and nomenclature of compounds and acids. A compound may be defined as a pure substance which contains more than one kind of atoms and/or ion. A compound may be chemically decomposed into simpler pure substances (eg. element).
Classification of Compounds
Compounds may be classified as ionic or molecular. This classification of compounds is emphasized by the different:
- Types of elements that combine to form ionic and molecular compounds.
- Types of chemical bonds within ionic and molecular compounds.
- Ways of naming and writing chemical formulas for ionic and molecular compounds.
- Physical and chemical properties of ionic and molecular compounds.
CHEMICAL BONDS
Classification of Chemical Bonds
For purposes of this unit, chemical bonds (binding forces) will be classified as covalent and ionic.
Covalent Bonds
A covalent bond is a force of attraction between two nonmetallic atoms. The two nonmetallic atoms both want to gain electrons to attain a noble gas electron arrangement. Since neither atom wants to lose electrons, they comprise by sharing electrons. The simultaneous attraction of electrons between two nuclei results in a force of attraction
COVALENT BONDING IN MOLECULAR SUBSTANCES
Covalent Bonding
For purposes of this course, covalent bonding occurs between nonmetallic atoms only. The sharing ofelectrons results in a noble-gas like electron structure (octet) around each atom. The force of attraction between the atoms (not ions) results from the simultaneous attraction of shared elec trons by two different nuclei.
Molecular Formulas
The neutral group of nonmetallic atoms covalently bonded together is called a molecule. The number of each kind of atom bonded together is represented by a chemical formula. The chemical formula of a molecule is called its molecular formula. A substance (either an element or a compound) which contains only nonmetallic atoms covalently bonded as molecules is called a molecular substance.
In Chem 20 a boding theory is presented which will explain why the molecular formulas are what they are (i.e. why hydrogen and oxygen bond to yield H2O not HO2). For Science 10 things are kept simple by either providing the name or formula for all molecular substances encountered.
Molecular Elements
Not all nonmetallic elements are molecular (i.e. exist naturally as distinct groups of atoms). However, all molecular elements are nonmetallic elements. A further classification of elements appears in Chem 20.
Nonmolecular Nonmetallic Elements |
Molecular Monoatomic Elements |
Molecular Diatomic Elements |
Other Molecular Elements |
C or Cn |
Noble gases |
Group VIIA |
P4 (white) |
Notes:
- Chemists and chemistry students find it very useful to memorize the formulas in the above table.
- In this course, white phosphorus (P4) is always assumed. Red phosphorus (which is the less dangerous type) would have a formula P or Pn.
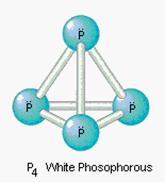
The formulas for the other nonmetallic elements (boron, arsenic, selenium, and tellurium) are complex and not required for Science 10.
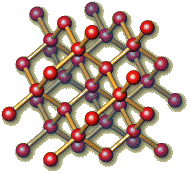
Diamond solid sulfur
(tetrahedral, Cn) (on eight membered ring, S8)
MOLECULAR COMPOUNDS
Binary Molecular Compounds
Binary molecular compounds are, as the name suggests, compounds, molecular, and binary. A compound consists of more than one kind of atom. A molecular compound consists of more that one kind of nonmetallic atoms. A binary molecular compound consists of only two kinds of nonmetallic atom. The majority of molecular compounds that are considered in Science 10 are binary molecular compounds. A system of nomenclature is provided below for binary molecular compounds. (The few nonbinary molecular compounds required in Science 10 will have to be memorized.)
The Prefix System of Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) recommends that molecular compounds should be named using the prefix system only (Section 2.252). A different system is recommended for ionic compounds and is described later in this unit. A very important objective of this unit is that students be able to differentiate between molecular and ionic compounds. One way to emphasize the distinction is to rigorously employ a different nomenclature system for molecular and ionic compounds.
In the prefix system, Greek or Roman prefixes are used to indicate the number of each kind of atom covalently bonded to one another (IUPAC Section 2.251). These prefixes should be learned for instant recall.
mono = 1 |
hexa = 6 |
di = 2 |
hepta = 7 |
tri = 3 |
octa = 8 |
tetra = 4 |
nona = 9 |
penta = 5 |
deca = 10 |
MOLECULAR SUBSTANCES
Prefix Examples |
Other Formulas to be Learned |
||
Formula |
Name |
Formula |
Name |
CO |
Carbon monoxide |
O3 |
Ozone |
N2O |
Dinitrogen monoxide |
HOH (H2O) |
Water |
SO3 |
Sulfur trioxide |
NH3 |
Ammonia |
PCl5 |
Phosphorus pentachloride |
CH4 |
Methane |
N2O4 |
Dinitrogen tetraoxide |
C12H22O11 |
sucrose |
|
|
CH3OH |
Methanol |
|
|
C2H5OH |
Ethanol |
|
|
H2O2 |
Hydrogen peroxide |
Notes:
- The first element in the formula should be named in full. The second element in the formula should be shortened and given an ide suffix (IUPAC, Section 2.21 and 2.22).
- "The prefix mono may generally be omitted". However, "extreme caution is advised in the omission of numerical prefixes, including mono" (IUPAC, Section 2.251).
- Hydrogen compounds are molecular. However, most common hydrogen compounds have trivial (vs. systematic) names. Some preferred trivial names are water for H2O (vs. dihydrogen oxide), ammonia for NH3 (vs. nitrogen trihydride) and hydrogen sulfide for H2S (vs. dihydrogen sulfide).
- No knowledge of covalent bonding capacity is required for Science 10. Either the name or formula will be given for any molecular substance used.
- From this point on it will be assumed that the formulas to be learned above have been memorized.
Worksheet #9 - Nomenclature of Molecular Compounds
Provide either the name or molecular formula in the following table as required.
Molecular formula |
Description or use (for interest only) |
Name |
||
e.g. CCl4 |
toxic cleaning fluid and solvent |
carbon tetrachloride |
||
1. |
|
composition of air |
78.03% |
nitrogen |
2. |
|
20.99% |
oxygen |
|
3. |
|
0.94% |
argon |
|
4. |
CO2 |
0.035% |
|
|
5. |
|
0.0016% |
other noble gases |
|
6. |
NO |
air pollutants |
In automobile exhaust |
|
7. |
NO2 |
Los Angeles - type smog |
|
|
8. |
|
London - type smog |
sulfur dioxide |
|
9. |
SO3 |
Becomes sulfuric acid |
|
|
10. |
|
Colorless and odorless poison |
carbon monoxide |
|
11. |
|
Good in upper atmosphere |
ozone |
|
12. |
|
grain alcohol, ethyl alcohol |
ethanol |
|
13. |
|
table sugar |
sucrose |
|
14. |
|
yellow solid in Group VIA |
sulfur |
|
15. |
P4O10 |
oxides formed by burning white phosphorus in air |
|
|
16. |
P4O6 |
|
||
17. |
|
chlorination of water |
chlorine dioxide |
|
18. |
|
methyl alcohol, methyl hydrate |
methanol |
|
19. |
|
a white solid |
phosphorus |
|
20. |
|
a cleaner when dissolved in water |
ammonia |
|
21. |
CH4 |
85 - 95% of natural gas |
|
|
22. |
HCl |
a gas; in water is hydrochloric acid |
|
|
23. |
|
laughing gas, anaesthetic |
dinitrogen oxide |
|
24. |
|
tincture of iodine in alcohol |
iodine |
|
25. |
H2O |
the most common solvent |
|
|
MOLECULAR MODELS
Purpose:
- To provide practice with the nomenclature for molecular substances.
2. To help visualize the three dimensional structure of molecules. (It is not the purpose of this lab to learn
the molecular structure of molecules nor to check the bonding capacity of nonmetallic atoms.)
Prelab Exercise:
Provide either the name or formula as required in the table below.
Procedure:
- Assemble molecular models for the species listed using the balls consistent with the color coding provided. When assembling always put the least plentiful atom (with the most bonds possible) at the center. For some models not all of the holes in the balls will be used. Nature is symmetrical; make the molecule as symmetrical s possible. (When assembling and disassembling models with springs, always twist clockwise (to the right) to avoid unravelling the spring.)
- Draw a 3D shape diagram for the molecule.
- Repeat Steps 1 - 2. Do not disassemble any of the models until the teacher has checked them.
- When finished, organize the balls properly in the box provided.
BINARY IONIC COMPOUNDS
Composition of Binary Ionic Compounds
A binary ionic compound is composed of metallic and nonmetallic ions. To qualify as binary only two kinds of simple ions must be involved. The metallic ions are positively charged. The nonmetallic ions are negatively charged. The attraction between the positive metallic ions and the negative nonmetallic ions is called an ionic bond. In the next unit, a study is made of how ionic compounds may be formed by simple composition, single replacement and double replacement reactions.
Structure of Binary Ionic Compounds
In the pure state, molecular compounds exist as molecules and ionic substances exist as ions. For example, molecules of sodium chloride do not exist. Sodium chloride exists as an ionic crystal lattice. Each sodium ion is surrounded by six chloride ions and each chloride ion is surrounded by six sodium ions. The ratio of sodium ions to chloride ions is 1:1. There are an equal number of sodium ions and chloride ions, but no one sodium ion is associated with one particular chloride ion.
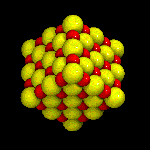
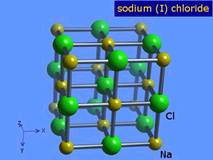
Ionic bonding could best be described as the simultaneous attraction of a positive ion (cation) by the surrounding negative ions (anions) and of a negative ion by the surrounding positive ions.
Molecules and Formula Units
Chemical formulas for molecular substances are called molecular formulas. Chemical formulas for ionic substances are called empirical formulas. The term chemical formula may be used for either molecular or empirical formulas.
Molecular substances contain molecules. Molecules are separate and discrete and neutral entities. A molecule of water may be identified as a group of 2 hydrogen atoms and one oxygen atom. Regardless of whether water is a solid, liquid, or gas, this group of atoms stays as an identifiable group.
Ionic substances do not contain molecules. There is no such thing as a molecule of sodium chloride. A formula unit of sodium chloride would contain one sodium ion and one chloride ion. As explained above, no one Na+ ion is attached to only one Cl - ion. Therefore, a formula unit is only an imaginary unit, which is an expression of the simplest whole number ratio of cations to anions. (Chemicals are not used on the basis of a small number of molecules or formula units. In the next unit, the introduction of the mole will remove most of the necessity for using the terms molecules and formula units.)
NOMENCLATURE OF BINARY IONIC COMPOUNDS
Naming Binary Ionic Compounds
When naming any ionic compound, the rule is to simply write the name of the cation (positive ion) followed by the name of the anion (negative ion).
In the first unit the rules for naming simple ions were given.
- Name the cation by writing the full name of the metallic element: e.g. Na+ is a sodium ion, Zn2+ is a zinc ion and Al3+ is an aluminum ion.
- Name the anion by abbreviating the full name of the nonmetallic element and adding ide; e.g. Cl - is a chloride ion, S2- is a sulfide ion and H - is a hydride ion.
Examples
NaCl or Na+Cl - is sodium chloride (table salt).
CaCl2 or Ca2+(Cl -)2 is calcium chloride (a drying agent).
(Is there chlorine in calcium chloride? __________)
Writing Empirical Formulas for Binary Ionic Compounds
- When given the name of a binary ionic compounds, first write the symbols for the ions involved.
Examples
For silver chloride write Ag+Cl -.
For potassium oxide write K+O2-.
For aluminum sulfide write Al3+S2-.
- Next determine the lowest whole number ratio of ions which will provide an overall net charge of zero
For silver chloride, Ag+Cl - becomes Ag+1Cl -1 or AgCl.
For potassium oxide, K+ O2- becomes (K+)2O2-1 or K2O.
For aluminum sulfide, Al3+S2- becomes (Al3+)2(S2-)3 or Al2S3.
Questions
Complete the following table.
Ionic Compound |
Total Positive Charge Per Formula Unit |
Total Negative Charge Per Formula Unit |
Net Charge Per Formula Unit |
|
1. |
Ag+Cl - |
|
|
|
2. |
(K+)2O2- |
|
|
|
3. |
(Al3+)2(S2-)3 |
|
|
|
Note: In math, the total charges would represent the lowest common multiple of the individual charges on the ions.
4. Should not the empirical formulas in the above table be AgCl2, KO2, and AlS8,
since chlorine is Cl2, oxygen is O2, and sulfur is S8 and all metals are
monoatomic? Explain.
- Students sometimes write Ba2O2 for barium oxide. What is wrong with this empirical formula?
WORKSHEET #10 - Compounds, Bonding and Nomenclature - Nomenclature of Binary Ionic Compounds
The following table contains binary ionic compounds where only one ionic charge is possible for each simple ion. The description or use of the compound is provided for information and interest only.
|
Chemical Formula |
Description or Use |
Name of Compound |
Example |
CaCl2 |
White solid; wetting agent |
calcium chloride |
1. |
|
Dietary supplement |
potassium iodide |
2. |
MgO |
White powder; magnesium ore |
|
3. |
|
antiperspirant |
aluminum chloride |
4. |
NaBr |
In Epsom salts |
|
5. |
Al2O3 |
Whiting; aluminum ore |
|
6. |
|
Black; lithium reacts with air |
lithium nitride |
7. |
CaO |
White powder; quicklime |
|
8. |
|
White solid like CaCl2 |
barium chloride |
9. |
|
White solid; table salt |
sodium chloride |
10. |
ZnO |
Protective oxide on zinc metal |
|
11. |
|
Photographic emulsion |
silver bromide |
12. |
|
Magnesium reacts with hydrogen |
magnesium hydride |
13. |
|
11% of minerals in sea water |
magnesium chloride |
14. |
|
In soldering paste |
zinc chloride |
15. |
Ag2S |
Argentite (silver ore) |
|
16. |
|
Potash (fertilizer) |
potassium chloride |
17. |
CaF2 |
Fluorite (pretty mauve crystals) |
|
18. |
|
For toning pictures brown |
sodium sulfide |
19. |
CaH2 |
Preparation of hydrogen |
|
20. |
|
Zinc blende (zinc ore) |
zinc sulfide |
NOMECLATURE INVOLVING ION CHARGES
Because of their electron arrangement, some metals form two or more different ions. For example, an iron atom may lose either two or three electrons to form either an iron (III) ion (Fe3+) or an iron (II) ion (Fe2+).
Two different naming systems may be used for compounds formed by metals which form ions of different charges. The preferred system is covered first - the Stock System.
The Stock System
The Stock System is named after Alfred Stock (1876 - 1946) and is the preferred system of the International Union of Pure and Applied Chemistry (IUPAC).
The name of the ion includes the charge on the ion as Roman numerals in parenthesis. See iron on the periodic table of ions. The mineral magnetite contains both types of iron oxides.
Formula of Ions |
Name of Ions |
Formula of Compound |
Name of Compound |
Fe3+ |
iron (III) |
(Fe3+)2(O2-)3 or Fe2O3 |
iron (III) oxide |
Fe2+ |
iron (II) |
Fe2+ O2- or FeO |
iron (II) oxide |
According to the periodic table of ions, which iron ion is the most common?_________
Always use the most common ion if neither is specified: i.e. "iron oxide" should be interpreted as iron (III) oxide.
The Classical (ic, ous) System
The Classical System of indicating ion charges is "not recommended" by IUPAC (Section 2.253). The Classical System is generally used only with elements which have a Latin symbol for the element. Examples from the periodic table of ions are iron (Fe), copper (Cu), tin (Sn), lead (Pb), and mercury (Hg). The suffix ic or ousis added to the Latin name to indicate reference to the larger (ic) and the smaller (ous) charge on the ion.
Formula of Ions |
Name of Ions |
Formula of Compound |
Name of Compound |
Fe3+ |
ferric |
(Fe3+)2(O2-)3 or Fe2O3 |
ferric oxide |
Fe2+ |
ferrous |
Fe2+ O2- or FeO |
ferrous oxide |
Note: The Stock and Classical Systems are only used when more than one ionic charge occurs. Do not use either system for naming ions where only one charge is given on the periodic table.
Example: ZnO is named zinc oxide not zinc (II) oxide.
Determining the Name for the Formula
When given the formula for a substance in which two or more ion charges are possible for the metal, work backwards from the charge on the negative ion to determine the charge on the positive ion.
Example: PbO2 Þ Pb(O2-)2 Þ Pb4+(O2-)2 Þ lead (IV) oxide
WORKSHEET #11 - Compounds, Bonding and Nomenclature - Nomenclature Involving Multiple Ion Charges
When naming compounds use the Stock System in preference to the Classical System. When given the chemical formula determine the ion charges first.
|
Chemical Formula |
Description or Use |
Name of Compound |
Example |
Cu2S |
Copper ore (chalcocite) |
copper (I) sulfide |
1. |
|
Uranium ore (uraninite) |
uranium (IV) oxide |
2. |
|
Lead ore (galena) |
lead (IV) sulfide |
3. |
SnO2 |
Tin ore (cassiterite) |
|
4. |
|
Manganese ore (pyrolusite) |
manganese (IV) oxide |
5. |
Sb2S3 |
Antimony ore (stibnite) |
|
6. |
|
Iron ore (hematite) |
ferric oxide |
7. |
HgS |
Mercury ore (cinnabar) |
|
8. |
MoS2 |
Molybdenum ore (molybdenite) |
|
9. |
|
Copper ore (chalcopyrite) |
copper (II) sulfide |
10. |
FeS |
Also in chalcopyrite |
|
11. |
|
Electrode in car battery |
lead (IV) oxide |
12. |
HgO |
Laboratory preparation of oxygen |
|
13. |
V2O5 |
A common catalyst |
|
14. |
|
Toothpaste additive |
stannous fluoride |
15. |
|
A green paint pigment |
chromic oxide |
16. |
TiO2 |
A white paint pigment |
|
17. |
AuCl3 |
Gold tinting of pictures |
|
18. |
|
Separating types of U atoms |
uranium (VI) fluoride |
19. |
NiBr2 |
Forms a green solution |
|
20. |
|
Forms a pink solution |
cobaltous chloride |
NOMENCLATURE INVOLVING COMPLEX IONS
Why Complex Ions Form
Complex ions may be thought of as groups of atoms which are made stable by sharing electrons, and which then become even more stable by gaining (usually) or losing electrons. Unlike neutral molecules, complex ions carry an electric charge and do not exist by themselves. The nitrate ions, NO3-, in a compound such as silver nitrate, AgNO3, exist as a group of one nitrogen and three oxygen atoms sharing electrons. The group has gained one electron to become more stable.
Formation of Compounds by Complex Ions
A complex is a very stable (strongly held together) group of atoms. Complex ions act like simple ions when forming compounds. An ionic bond is formed by the attraction of a positive simple ion to a negative complex ion or of a positive complex ion (NH4+) to a negative complex ion. The compound formed is called an ionic compound. As for all ionic compounds, the total positive charge in the formula must be equal to the total negative charge. The names and charges of many common complex ions are given on the periodic table.
Note that some complex ions have two names listed, such as HCO3- (hydrogen carbonate or bicarbonate)
In such cases, the Science 10 course will assume recognition of the ion by either name. The first name is preferred by IUPAC and should be used when names of compounds are to be written by students.
The hypochlorite ion is commonly found with its formula given as ClO - or as OCl -. Again, this course will assume recognition of either form, but use the ClO - form when compound formulas are to be written by students.
Examples:
- Sodium ions and carbonate ions bond ionically to form an ionic compound.
Na2CO3 - sodium carbonate (washing soda)
- Ammonium ions and hydrogen phosphate ions bond ionically to form an ionic compound.
(NH4)2HPO4 - ammonium hydrogen phosphate (a fertilizer)
- Magnesium ions and hydroxide ions bond ionically to form an ionic compound.
Mg(OH)2 - magnesium hydroxide (milk of magnesia)
Note that when placing a subscript number after the formula for a complex ion, the group formula is first bracketed.
Naming Complex Ions
Complex ions usually remain as stable groups in chemical reactions, although they may become part of different compounds. Complex ions are assigned special names.
The most common forms of complex ions are given the suffix ate. The naming of the variations from the most common employ a system of prefixes and suffixes which are given on the periodic table.
The following exercise will provide some practice in naming complex ions. (Remember that complex ions are not molecules, they cannot exist by themselves as they are in this exercise.)
|
Ion Name |
Formula |
|
Ion Name |
Formula |
1. |
bisulfate |
|
6. |
sulfite |
|
2. |
|
ClO4 - |
7. |
|
NO3- |
3. |
|
NH4+ |
8. |
hydrogen sulfide |
|
4. |
dichromate |
|
9. |
|
HPO42- |
5. |
|
OH - |
10. |
|
CH3COO - |
WORKSHEET #12 - Compounds, Bonding and Nomenclature - Nomenclature Involving Complex Ions and Others
The following exercise primarily involves nomenclature of ionic compounds containing complex ions. A couple of molecular compounds also appear in the exercise. Use the table of complex ions on Side 2 of the ALCHEM periodic table to answer the following questions. Only those complex ions listed on the ALCHEM periodic table are used. Classify each substance as ionic (I) or molecular (M).
|
I or M |
Chemical Formula |
Name of Compound |
1. |
|
K2CO3 |
|
2. |
|
(NH4)2S |
|
3. |
|
|
calcium hydroxide |
4. |
|
|
magnesium silicate |
5. |
|
|
iron (II) chlorite |
6. |
|
Cr(NO3)3 |
|
7. |
|
|
potassium dichromate |
8. |
|
SO3 |
|
9. |
|
NaNO2 |
|
10. |
|
|
ammonium sulfate |
11. |
|
|
sodium bicarbonate |
12. |
|
K3PO4 |
|
13. |
|
|
calcium stearate |
14. |
|
NH3 |
|
15. |
|
|
sodium nitrate |
16. |
|
KMnO4 |
|
17. |
|
|
sodium thiosulfate |
18. |
|
CO2 |
|
19. |
|
|
barium perchlorate |
20. |
|
|
sodium hydrogen sulfide |
21. |
|
|
potassium cyanide |
22. |
|
NH4H2PO4 |
|
23. |
|
|
sodium glutamate (MSG) |
24. |
|
Na2SO4 |
|
25. |
|
|
potassium thiocyanate |
WORKSHEET #13 - Compounds, Bonding and Nomenclature - Hydrated Compounds
A number of ionic compounds called hydrates produce water when they decompose upon heating. When the formula of a hydrated compound is written, the number of water molecules is also included. For example, the formula for copper (II) sulfate pentahydrate is written as CuSO4·5H2O. The name for CuSO4·5H2O is copper (II) sulfate pentahydrate indicating that five molecules of water are bonded within the ionic crystal for every one formula unit of CuSO4. The prefixes in the following table are used to indicate the number of water molecules in a hydrated compound. These are the same prefixes as used previously to name molecular compounds.
Mono = 1 |
Hexa = 6 |
Di = 2 |
Hepta = 7 |
Tri = 3 |
Octa = 8 |
Tetra = 4 |
Nona = 9 |
Penta = 5 |
Deca = 10 |
|
Name of Hydrate |
Common Name, Use or Description |
Formula |
E.g. |
copper (II) sulfate pentahydrate |
Blue vitriol, bluestone, copper plating, blue solid |
CuSO4·5H2O |
1. |
|
Epsom salts, white solid explosives, matches |
MgSO4·7H2O |
2. |
sodium carbonate decahydrate |
Washing soda, soda ash, water softener, white solid |
|
3. |
|
White solid, fireproofing wood, disinfectants, parchment paper |
MgCl2·6H2O |
4. |
barium chloride dihydrate |
White solid, pigments, dyeing fabrics, tanning leather |
|
5. |
|
White solid, photographic emulsions |
Cd(NO3)2·4H2O |
6. |
|
White solid, embalming material, fireproofing lumber, vulcanizing |
ZnCl2·6H2O |
7. |
zinc sulfate heptahydrate |
White solid, clarifying glue, preserving wood and skins |
|
8. |
lithium chloride tetrahydrate |
White solid, soldering aluminum, in fireworks |
|
9. |
|
Photographic hypo, antichlor, white solid |
Na2S2O3·5H2O |
10. |
cobalt (II) chloride hexahydrate |
Pink solid, humidity and water indicator, foam stabilizer in beer |
|
11. |
|
White solid, antiperspirant |
AlCl3·6H2O |
12. |
|
De-icer used on icy highways, added to cement mixtures to prevent freezing during winter, white solid |
CaCl2·2H2O |
13. |
barium hydroxide octahydrate |
White solid, manufacture of glass, softener |
|
14. |
nickel (II) chloride hexahydrate |
Green solid, absorbent for ammonia in gas masks |
|
15. |
|
Glauber's salt (a medicine), white solid, drying agent |
Na2SO4·10H2O |
THE HISTORY OF CHEMICAL NOMENCLATURE

Antoine Laurent Lavoisier (1743 - 1794)
The modern system of naming compounds according to the elements from which they are formed dates back to 1789 when Lavoisier published a chemistry book using a new nomenclature. Lavoisier was the first chemist to clearly understand the oxidations of elements. For example, in the reaction
Nitrogen + oxygen Þ nitrogen dioxide
Two elements combine to form a compound. To us this statement is quite clear but prior to Lavoisier's book, the reaction was written:
Mephitic air (nitrogen) + dephlogisticated air (oxygen) Þ brown gas (nitrogen dioxide)
Lavoisier in 1789 introduced the following ideas:
- All elements have single word names.
- All compounds have names made up of the elements.
- A system of prefixes and suffixes should be used.
Common Name |
Systematic Name |
fixed air |
carbon dioxide |
cinnabar |
mercuric sulfide |
John Dalton (1766 - 1844)
John Dalton, an English school teacher, proposed a theory that explained chemical reactions. He proposed that:
- All substances are made up of atoms.
- Atoms of one substance are alike and have the same mass.
- Chemical action consists of a rearrangement of atoms.
- Elements are made up of "simple atoms" while compounds contain "compound atoms" (now called molecules)

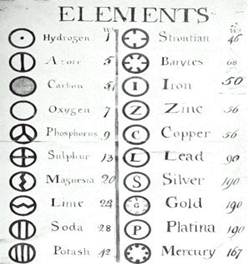
To help in the writing of chemical equations, Dalton, over the period 1806 - 1835, devised a symbol for each known element. Note that some things thought to be elements are not in fact elements.
Dalton's symbols could be used to represent the chemical formulas of substances and to write chemical equations.
Source : http://www.archbishopjordan.ab.ca/Science%20Notes/Science_10/Sci10Chem.doc
Web site link: http://www.archbishopjordan.ab.ca
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Chemistry notes
Chemistry notes
Chemistry notes
This is the right place where find the answers to your questions like :
What is Chemistry notes? What does Chemistry notes mean ? Which is the meaning of Chemistry notes?
Chemistry notes chemistry notes
Alanpedia.com from 1998 year by year new sites and innovations
Main page - Disclaimer - Contact us