Heat
Heat
The following text is used only for teaching, research, scholarship, educational use and informative purpose following the fair use principles.
We thank the authors of the texts and the source web site that give us the opportunity to share their knowledge
Physics
Heat
Student Notes
Temperature is a measure of the hotness or coldness of an object
Heat is a form of energy and it can be converted into other forms of energy
To show that heat is a form of energy you must be able to show that it can do work (because energy is the ability to do work). Work is done when an object is being moved. So we need to show that heat can move something.
Demonstration
Alcohol in a thermometer moves up the glass when heat is applied.
Solids, liquids and gases expand when heated, and contract when cooled
Solids expand when heated and contract when cooled
Demonstration
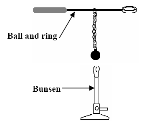
 Heat the brass ball.
Heat the brass ball.- Note that he ball fits through the ring when the ball is cold but not when hot.
Liquids expand when heated and contract when cooled
Demonstration
- Connect a glass tube to the top of a beaker of water (use dye to make the water more visible). I use a Bunsen burner instead of a hair-dryer).
- Note that the water rises up the tube as it gets heated and drops back down as it cools.
Gases expand when heated and contract when cooled
Demonstration
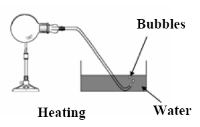
- Gently heat the flask of air (much better to use a hair dryer in case the flask breaks).
- Note that bubbles come out of the tube when the flask is heated and as it cools water from the trough rises back up the tube because of the partial vacuum which has formed.
The anomaly (strangeness) of water
Water is an exception to almost all other substances in that when it drops below 4 0C the water actually expands when cooling rather than contracting.
The explanation for this is a little complicated (it has to do with the arrangement of water molecules, but you don’t need to know it for exam purposes).
Demonstration
- Fill a glass bottle with water and place it in a plastic bag in a freezer.
- When you take it out the following day the bottle will be broken because the water has expanded on freezing.
(The purpose of the bag was to ensure that all the pieces of glass get taken out).
This is why water pipes sometimes burst in winter causing flooding in a home.
The effect of pressure on the boiling point of water
Reduced pressure decreases the boiling point of water
Demonstration
- Suck up water which is at about 80 0C into the syringe so that the syringe is about one-quarter full.
- Cover the open end (watch out- it’s hot!) and pull back the handle to create a partial vacuum.
- Result: The water begins to boil!
- Explanation: the air acts like a blanket which presses down on the water and makes it difficult for the water molecules to leave (‘jump out of’) the liquid and become part of the air.
- Higher pressure therefore results in a higher boiling point (the molecules need to have more energy/ move more rapidly to make the transition).
Changes of state, the cooling curve and latent heat
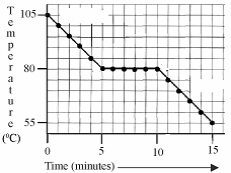
- Heat a boiling tube of wax to a high temperature and as it cools note the temperature.
- Plot a graph of temperature against time.
- Note that the temperature decreased at a steady rate until (in this case) it reached 80 0C.
- This is when the wax began to change state from a liquid to a solid.
- It remains at this temperature until all the wax has solidified (in this case it took 5 minutes) and after that it began to drop in temperature again as the solid wax cooled down.
Explanation
As the wax changes state from a liquid to a solid it gives out heat without cooling down. This heat is called latent heat because latent means ‘hidden’ and in this case it is not obvious where the heat is coming from.
Latent heat is the heat taken in or given out when a substance is changing state (without changing temperature)
Heat Transfer: Conduction, Convection and Radiation
Heat can be transferred in three different ways - conduction, convection and radiation
Conduction is the method by which heat travels from particle to particle through a solid
Convection is the transfer of heat through a liquid or gas when the particles move and carry the heat with them
Radiation is the transfer of heat from a hot object without the need for a medium
All objects radiate heat, but not all substances conduct or convect heat
|
Conduction |
Convection |
Radiation |
Solids |
Yes |
No |
Yes |
Liquids |
No |
Yes |
Yes |
Gases |
No |
Yes |
Yes |
 Conduction
Conduction
To compare the ability of different metals to conduct heat
- Use the apparatus shown which consists of a piece of timber with four different strips of metal.
- Place some candle wax at the end of each metal and stand a match in the wax at the end.
- Light the Bunsen (or candle) under the middle and note the order in which the matches fall.
The match which falls first was standing in the best conductor.
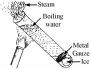
To show that water is a poor conductor of heat
- Half fill a boiling tube with water and use piece of metal gauze to hold down the ice.
- Holding the boiling tube at an angle with a tongs, heat it at the top using the Bunsen burner for a short period of time.
Result: the water at the top boils which the ice at t he bottom stays frozen.

Convection
To demonstrate convection currents in water
- Use the apparatus shown and drop in some copper sulphate or potassium permanganate to act as a dye.
- Place the Bunsen under one of the corners and note the movement of the water around the apparatus.
 To demonstrate convection currents in air
To demonstrate convection currents in air
Cut tissue paper into narrow strips; tie the strips together at one end using the piece of thread and hang them from a retort stand as shown over a hot-plate.
Result: the tissue paper will begin to move as a result of the convection current generated by the hot-plate.
Radiation
Dark materials are better radiators of heat than shiny materials.
Demonstration
- Take two identical metal containers and paint one with one black and the other silver.
- Fill both with hot water.
- Using a thermometer and stop-watch note which container cools the quickest.
- The dark container cooled more quickly because it is a better radiator of heat.
Conductors and Insulators
A conductor is a substance which allows heat to flow through it easily
An insulator is a substance which does not allow heat to flow through it easily
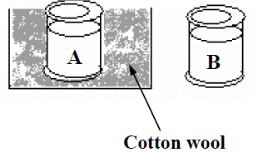
To compare the insulating ability of different materials
Demonstration
- Take two identical containers and wrap one in cotton wool.
- Fill both with hot water.
- Note which container cools more quickly than the other.
- The container which cooled more slowly had better insulating material.
Note: There are no maths problems in this chapter.
Exam Questions
- [2006]
Define temperature and give a unit used to express temperature
- [2008][2010] Give two differences between heat and temperature.
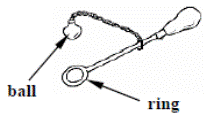 [2010 OL]
[2010 OL]
The diagram shows a piece of equipment that can be used to investigate the effect of heat on a metal.
The ball will pass through the ring when it is cold.
When the ball is heated it will no longer pass through the ring.
Answer the following questions about this investigation.
- Explain why the ball does not pass through the ring when it is heated.
- How would you get the ball to fit through again?
- What does this investigation tell us about the effect of heat on metals?
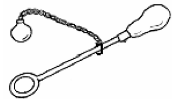 [2007 OL]
[2007 OL]
The apparatus drawn consists of a ball and ring.
When the ball and the ring are cold the ball just fits through the ring.
When the ball is heated the ball does not pass through the cold ring.
- What conclusion would you draw from this experiment?
- What would you expect to happen if the ball was cooled down again?
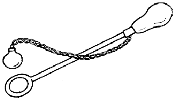 [2007]
[2007]
The diagram shows a “ball and ring” apparatus.
When the ball and ring are both cold the ball just passes through the ring.
How would you use this apparatus to show
- the expansion of a solid on heating
- the contraction of a solid on cooling?
- [2009 OL]
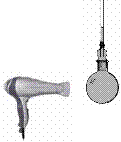 Describe, with the help of a labelled diagram, how you could carry out an experiment to show that metals expand when heated.
Describe, with the help of a labelled diagram, how you could carry out an experiment to show that metals expand when heated.
Use the following headings: Labelled diagram, Equipment, Procedure, Result.
- [2008 OL]
The diagram shows a round-bottomed flask full of coloured water.
- What would you expect to notice if the flask is heated gently?
- Give a reason why this should happen.
- Why is coloured water used during this investigation?
- [2010 OL]
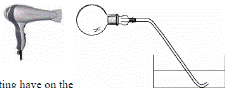 The picture shows a round-bottomed flask filled with air being heated gently with a hairdryer.
The picture shows a round-bottomed flask filled with air being heated gently with a hairdryer.
- What effect does the heating have on the volume of air in the flask?
- What would you expect to notice in the dish of water?
 [2007 OL]
[2007 OL]
In an investigation to see the effect heating had on gases, a student heated a round-bottomed flask containing air using a hairdryer as shown in the diagram.
- What would you expect the student to have seen when the flask was heated?
- What conclusion can you draw from this investigation?
 [2010]
[2010]
The apparatus shown in the diagram was used to investigate the expansion and contraction of a gas.
- What is observed when the flask is heated?
- Explain your observation when the flask is heated?
- What is observed when the flask is allowed to cool?
- Explain what you observe as the flask cools.
- [2006 OL]
Heat may be transferred from hot to cold places by the three methods listed on the right.
CONDUCTION CONVECTION RADIATION |
Choose the method of heat transfer that occurs in each of the following.
- The boiling of water in a kettle. ___________________________
- The heating of the Earth by the Sun. ___________________________

- [2006]
Describe an experiment to show the expansion of water when it freezes.
You may include a labelled diagram if you wish.
- [2009]
The boiling point of water can be determined using the apparatus shown in the diagram.
- Why are boiling (anti-bumping) chips added to the water?
- At what temperature does water boil, at standard (normal) atmospheric pressure?
- What effect does the raising of pressure have on the boiling point of water?
- What effect does the lowering of pressure have on the boiling point of water?
Conduction, Convection and Radiation
Conduction Convection Radiation |
- [2007 OL]
Heat is transferred in different ways.
 In each case use a word from the list on the right to correctly complete each sentence below.
In each case use a word from the list on the right to correctly complete each sentence below.
- Heat travels through solids by ___________________.
- Heat travels through liquids and gases by ___________________.
- Heat travels from the Sun to the Earth by ___________________.
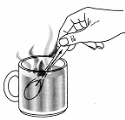
- [2006]
Name the mode of heat transfer from the hot liquid, through the spoon, to the hand.
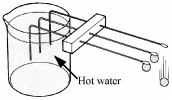
- [2009]
Copper, aluminium and iron rods are set-up as shown in the diagram. A metal ball is attached by wax to the end of each rod. Hot water is poured into the beaker. The ball falls from the copper rod first. What conclusion can be drawn from this observation?
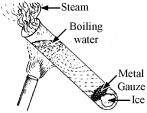 [2007]
[2007]- What does the experiment shown in the diagram tell us about the transfer of heat energy in water?
- If you wanted to warm all of the water why would the bottom of the test tube be the best place to heat with the Bunsen flame?
- [2006]
Heat moves in liquids by convection. Give one difference between convection and conduction.
 [2009]
[2009]
The photograph shows a solar panel being installed. Water passing through the panel is heated by the sun.
- How does heat from the sun travel, through the vacuum of space, to the earth?
- Give one advantage or one disadvantage of fitting solar panels to your home?
Insulation
- [2009 OL]
The diagram shows two metal cans equal in size and filled with the same amount of water at 100 °C. Can A is wrapped in cotton wool and can B has no wrapping.
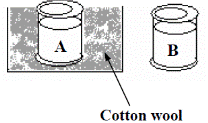 After 15 minutes, which can, A or B, would you expect to have the higher temperature?
After 15 minutes, which can, A or B, would you expect to have the higher temperature? - Give a reason for your answer.
Latent Heat
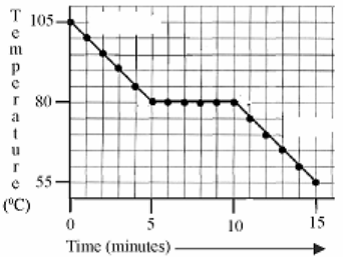 [2006]
[2006]
The graph is a cooling curve. The substance used in this experiment was naphthalene. Naphthalene has a melting point of 80 0C.
The rate of heat loss was constant throughout the experiment.
- What is happeningto the naphthalene on the horizontal section of the graph?
- What is the heat loss on the horizontal section of the curve called?
- [2010]
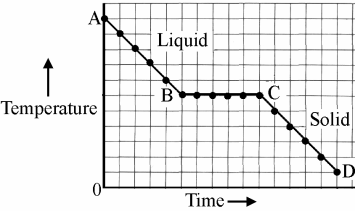 A substance that is a solid at room temperature was heated above its melting point and then allowed to cool at a steady rate. The temperature was taken at regular intervals. The data is in the graph.
A substance that is a solid at room temperature was heated above its melting point and then allowed to cool at a steady rate. The temperature was taken at regular intervals. The data is in the graph.
Why is there no drop in temperature between B and C?
- [2008]
A pupil heated some lauric acid, which is a solid at room temperature, until it turned into a liquid.
The lauric acid was then allowed to cool at a uniform rate. The temperature of the lauric acid was taken every minute.
The data from this experiment is given in the table.
Temperature (0C) |
75 |
64 |
54 |
43 |
43 |
43 |
43 |
43 |
32 |
22 |
10 |
Time (minutes) |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
 Draw a graph, using this data, of temperature against time (x-axis) in the grid provided below.
Draw a graph, using this data, of temperature against time (x-axis) in the grid provided below.
- Explain the shape of the graph that you obtain.
- Use the graph to estimate the melting point of lauric acid.
Exam Solutions
- Temperature is a measure of the hotness of an object.
The unit of temperature is degrees Celsius (0C).
- Heat is a form of energy (and temperature isn’t).
Temperature is a measure of the hotness of an object (and heat isn’t).
- Ball expanded / increased in volume
- Cool the ball / stated cooling method
- (Metals) expand when heated / contract when cooled
- Solids (metals) expand (when heated) / ball expands (when heated)
- It would fit through the ring / contracts / get smaller
- Heat the ball, it does not pass through the ring.
- Let the ball cool, it now passes through the ring again.
- Ball and ring apparatus as shown.
Ball fits through ring when both are at room temperature.
Heat the ball over a Bunsen burner for one minute.
Result: the ball will no longer fit through the ring.
- Water rises up the tube
- Water (liquid) expands (when heated)
- Easier to see
- Expands / increases
- Bubbles
- Bubbles of air coming from the mouth of the flask into water trough
- Air (gas) expands when heated
- Bubbles of air come out of the bottom of the glass tube.
- The air in the flask expanded.
- The bubbles stop and water rises up the glass tube.
- Air in flask contracted therefore the air pressure is less than atmospheric pressure.
- Convection
- Radiation
- Fill a bottle with water
Put the bottle in a freezer
The bottle bursts after a few hours.
- Chips help to ensure that all the water boils at the same temperature{I know – not on the syllabus so shouldn’t have appeared!}
- 1000 C
- It raises the boiling point
- It lowers the boiling point
Conduction, Convection and Radiation
-
- Conduction
- Convection
- Radiation
- Conduction
- Copper is the best conductor
- Water is a poor conductor of heat
- Because hot water rises (note that ‘heat rises’ alone gets no marks)
- Convection: particles of liquid move carrying the heat with them.
Conduction: heat is transferred from one particle to another without any overall movement of the particles themselves.
- It travels by radiation.
- Advantage: to reduce fuel bills, reduce CO2 emissions, it is renewable.
Disadvantage: expensive to set up, less heat is absorbed in winter or on cloudy days.
- Can A will have a higher temperature after 15 minutes.
- Because it is insulated so it loses heat more slowly.
Latent Heat
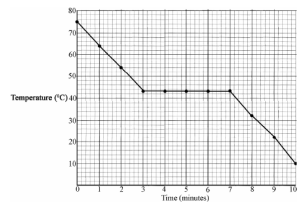 It is changing from a liquid to a solid
It is changing from a liquid to a solid - Latent heat of fusion
- It is changing state (freezing).
- See graph
- Initially the temperature of the liquid falls until it reaches 43 0C.
Here it changes state from a liquid to a solid.
Then the solid cools down.
- 43 0C
Heat Questions
- Draw a labelled diagram of an apparatus used to demonstrate convection in a liquid.
- Draw a labelled diagram of the apparatus used to compare the conductivity of different metals. How does it work?
- Give two uses of a bimetallic strip.
- Draw a labelled diagram of the apparatus you would use to show that water is a poor conductor of heat.
- How would you demonstrate that solids expand when heated and contract when cooled? Include a fully labelled diagram.
- How would you demonstrate that liquids expand when heated and contract when cooled? Include a fully labelled diagram.
- How would you demonstrate that gas expands when heated and contract when cooled? Include a fully labelled diagram.
- Why is water not a suitable liquid for use in thermometers?
- Define conduction.
- Define convection.
- A student filled a boiling tube with water and placed an ice-cube at the bottom by keeping metal weight on top of it. The student then heated the top of the boiling tube until the water was boiling at the top.
- What was the student trying to investigate?
Object |
Method of Heat Transfer |
A spoon |
|
A saucepan of water |
|
Space |
|
- Why did he put a weight on the cube of ice?
- Why was it important that the weight was made of metal?
- What did the student notice?
- Fill in the table with the words convection, conduction, or radiation where appropriate
- Why is the heating element of a kettle placed near the bottom?
- What is the main way in which heat is transferred when the water in an electric kettle is heated?
- What is the main way in which heat is transferred when the Sun heats the Earth?
- What property of oven gloves allow a baker to pick up hot bread?
- Why do metals expand when heated?
- What is meant by the term ‘the anomaly of water’?
- Sublimation occurs when a ________ changes directly to a ________ when heated.
- What is meant by the term ‘latent heat’?
- Draw a diagram of a cooling curve for wax and indicate on it all the various states of matter.
- Why are ice cubes much better at cooling a drink that the equal amount of iced water?
- Why is a scald from steam much more serious that a burn from boiling water?
- Why do hurricanes pick up energy when they pass over oceans?
- Why does a glass beaker often crack when you pour in boiling water?
- How come a shiny material is both a bad absorber of heat and a bad radiator of heat?
- How come when I do this experiment I don’t get a nice graph like this?
Higher Order Questions
- When stepping out of bed on a cold morning, why does it feel colder if your feet touch say, a marble floor rather than a floor with carpet even though both are at the same temperature?
- How does perspiration (sweating) help to keep us cool?
- Many Arabs wear dark clothing in warm weather, even though dark clothes are better at absorbing heat than white clothes. Any idea why?
- Heat can be transferred by conduction, convection or radiation. A thermos flask tries to keep hot liquids hot by preventing heat loss. Any idea how it minimises each of these three methods of heat transfer?
- What is the effect of increased pressure on the boiling point of water
- Why does increased pressure affect the boiling point of water?
Can you think of an application of this (a device which is built on this principle?
Can you think of anyone who might be affected by this?
Hurricanes pick up energy while travelling over the ocean.
Water evaporates and in doing so picks up heat energy when going from a liquid to a gas.
Now later on when it condenses from a gas to a liquid it gives out this heat in the form of molecular energy to nearby molecules which presumably heats them up.
Heat Crossword
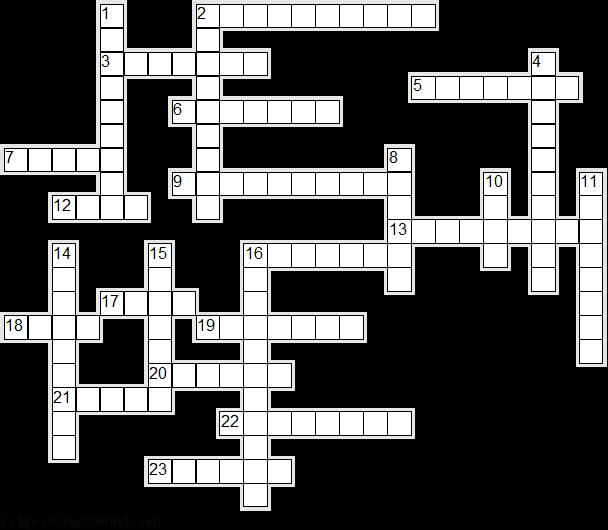
Across
2. Gas changing to liquid. (10)
3. Apparatus used to demonstrate that reduced pressure decreases the boiling point of water. (7)
5. You find this in most school thermometers. (7)
6. Visible evidence that gas expands when heated.(7)
7. A glass bottle of water might do this when the temperature drops below 4 degrees. (5)
9. Method by which heat travels from particle to particle through a solid. (10)
12. What type of materials are good absorbers of heat? (4)
13. Transfer of heat from a hot object without the need for a medium. (9)
16. Liquid changing to gas. (7)
17. What type of conductor of heat is water? (4)
18. Energy is defined as the ability to do this. (4)
19. Solid changing to liquid. (7)
20. The heat taken in or given out when a substance is changing state (without changing temperature). (6)
21. What type of materials are poor radiators of heat? (5)
22. Liquid changing to solid. (8)
23. Solids, liquids and gases do this when heated. (6)
Down
1. It is a substance which does not allow heat to flow through it easily. (9)
2. It is a substance which allows heat to flow through it easily. (9)
4. Transfer of heat through a liquid or gas when the particles move and carry the heat with them. (10)
8. Heat is a form of this. (6)
10. It's a form of energy. (4)
11. Solids, liquids and gas do this when cooled. (8)
14. The effect of increased pressure on the boiling point of water. (9)
15. The strangeness of water. (7)
16. used to demonstrate that solids expand when heated. (11)
From Puzzlemaker.com
M C P M I C A A S O W M G K G
X L O Q K L D X Y T G Y T V M
S I O N C Z F U R M S N Y A B
M B R O D D E L I O P R U V U
B G H G N U C R N U W L U O L
A O S A N O C B G I W F K B O
L Q S R T J W T E J C A C O M
N W Q F L U A D I A P K S E Q
W T E N R A G X Y O I N T U U
C O N D E N S I N G N E Q O I
C L S L J F D F G I U O M W N
V U F C K H B P Y U M Y L U F
U W Y O M X X A M W B O A D Y
Z S O P O T G E Y I Q D H A P
E S E L B B U B P V D Y M B B
ALCOHOL
BUBBLES
BURST
CONDENSING
CONDUCTION
SYRINGE
Source : http://www.thephysicsteacher.ie/JC%20Science/JC%20Physics/Student%20Notes/11.%20Heat.doc
Web site link: http://www.thephysicsteacher.ie
Author : not indicated on the source document of the above text
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship (for fair use as indicated in the United States copyrigh low) please send us an e-mail and we will remove your text quickly.
Heat
Heat
Heat
This is the right place where find the answers to your questions like :
Who ? What ? When ? Where ? Why ? Which ? How ? What does Heat mean ? Which is the meaning of Heat?
Heat physics notes
Alanpedia.com from 1998 year by year new sites and innovations
Main page - Disclaimer - Contact us